WHAT SHOULD I KNOW ABOUT MEDICAL CANNABIS?
The cannabis plant belongs to the family Cannabaceae, genus Cannabis.

Chemotypes
Cannabis cultivars can be classified in different chemotypes, generally depending on the relative amounts of THC and CBD (as percentage of weight) contained in the dried flowers.9 Cannabis chemotypes are:

THC-rich cannabis cultivars with little amounts of CBD (e.g. THC:CBD ratio 20:1)

Chemotype 2: cannabis cultivars containing a similar ratio of THC and CBD (ratio THC:CBD 1:1)

cannabis cultivars rich in CBD and small amounts of THC (e.g. ratio of THC:CBD 1:20)
The endocannabinoid
The endocannabinoid system (ECS) is a lipid signaling system that serves important regulatory functions in the whole organism favoring homeostasis.10, 21 This system modulates many physiological processes including the regulation of neurotransmission, as well as endocrine and paracrine actions (see figure below):

Sleep-wake cycle

Psychomotor behavior

Bone development and density

Pain perception

Emotional state

Skin

Cardiovascular system

Brain system

Digestive system

Immune system
The main components of this cellular communication system are:
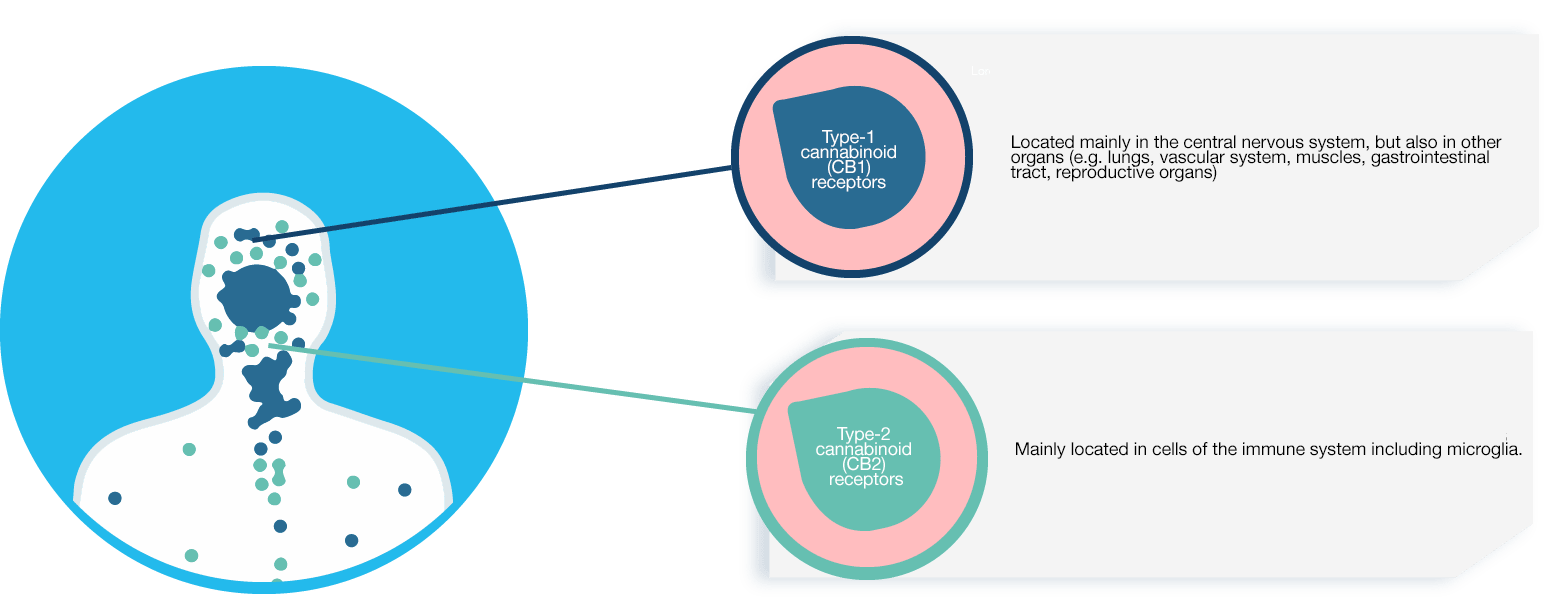
1. Cannabinoid receptors
2. Endocannabinoids
(endogenous ligands of cannabinoid receptors, derived from arachidonic acid)

AEA
N-archidonoylethanolamide, or “anandamide” (AEA), named after the Sanskrit term “Ananda”, which
means “internal bliss”.

2-AG
2-Archidonoylglycerol (2-AG).
3. Enzymes responsible for endocannabinoid synthesis

NAPE
Phospholipase D (NAPE-PLD) which produces anandamide and other fatty acid amides
such as OEA or PEA.

DAGL
Diacylglycerol lipase (DAGL) which synthesizes
2-AG
4. Enzymes responsible for endocannabinoid deactivation

FAAH
Fatty acid amido-hydrolase (FAAH) which hydrolyzes AEA and related fatty-acid ethanolamides (OEA and PEA)

MAGL
Monoacylglycerol lipase (MAGL), which degrades
2-AG.
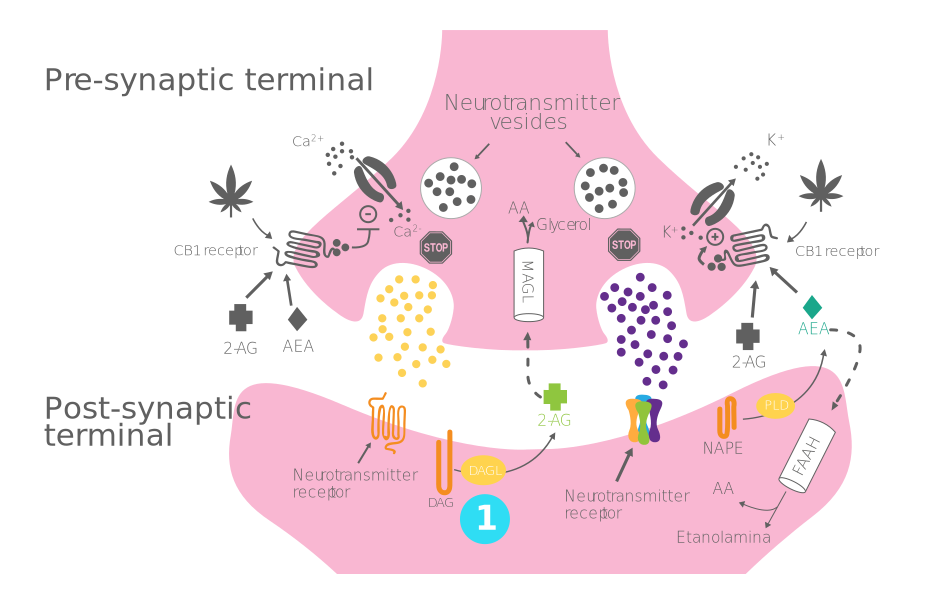
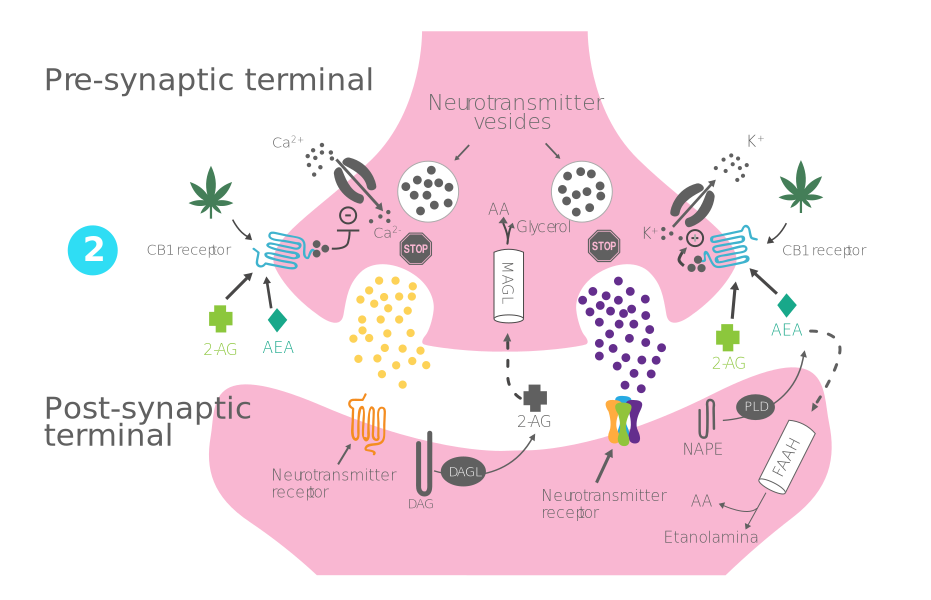
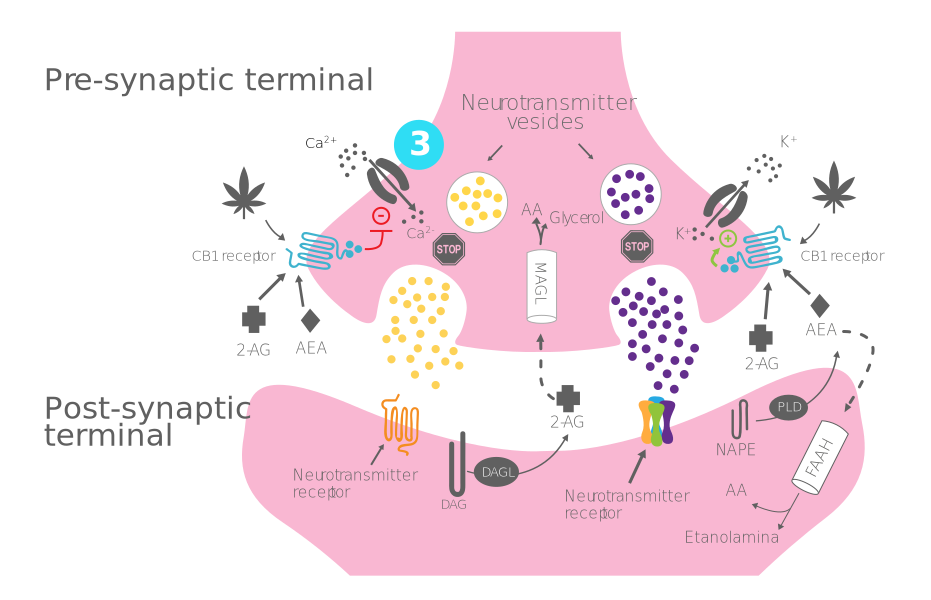
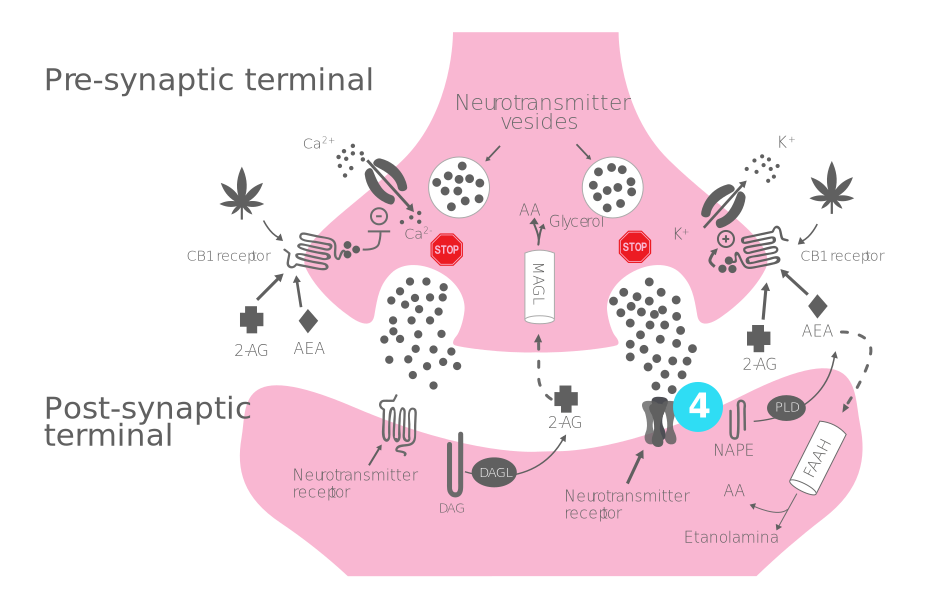
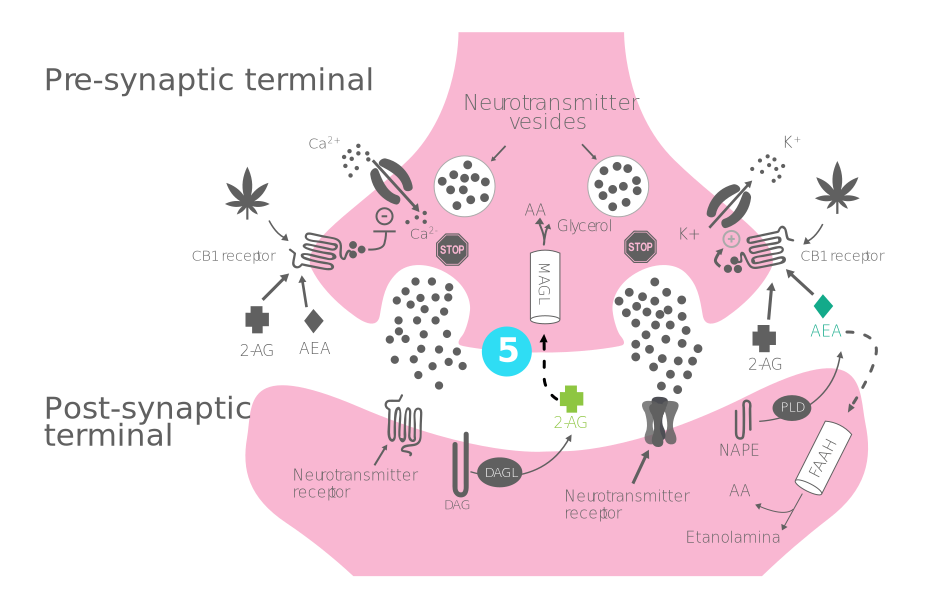
Process of production, release, and action of endocannabinoids:
- Step 1 - Synthesis of endocannabinoids: 2-AG and AEA are synthesized and released "on demand" by DAGL and NAPE-PLD from the postsynaptic terminal.
- Step 2 - Bonding to the CB1 receptor: 2-AG/AEA crosses the synaptic cleft to bind to CB1 receptors in the presynapsis.
- Step 3 - Opening of potassium channels and closure of calcium channels: Activation of CB1 receptors causes the inflow of K+ and the closure of Ca+ channels.
- Step 4 - Hyperpolarization of the presynaptic terminal and inhibition of the release of excitatory and inhibitory neurotransmitters
- Step 5 - Entry of endocannabinoids into the neuron and enzymatic degradation: 2-AG and AEA are degraded by MAGL and FAAH, respectively, inside the cell.